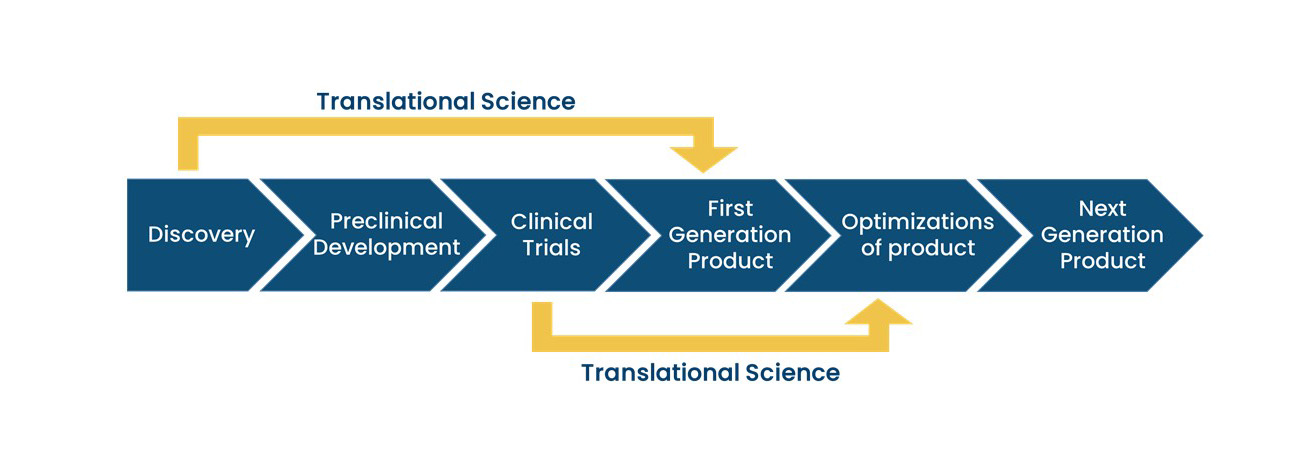
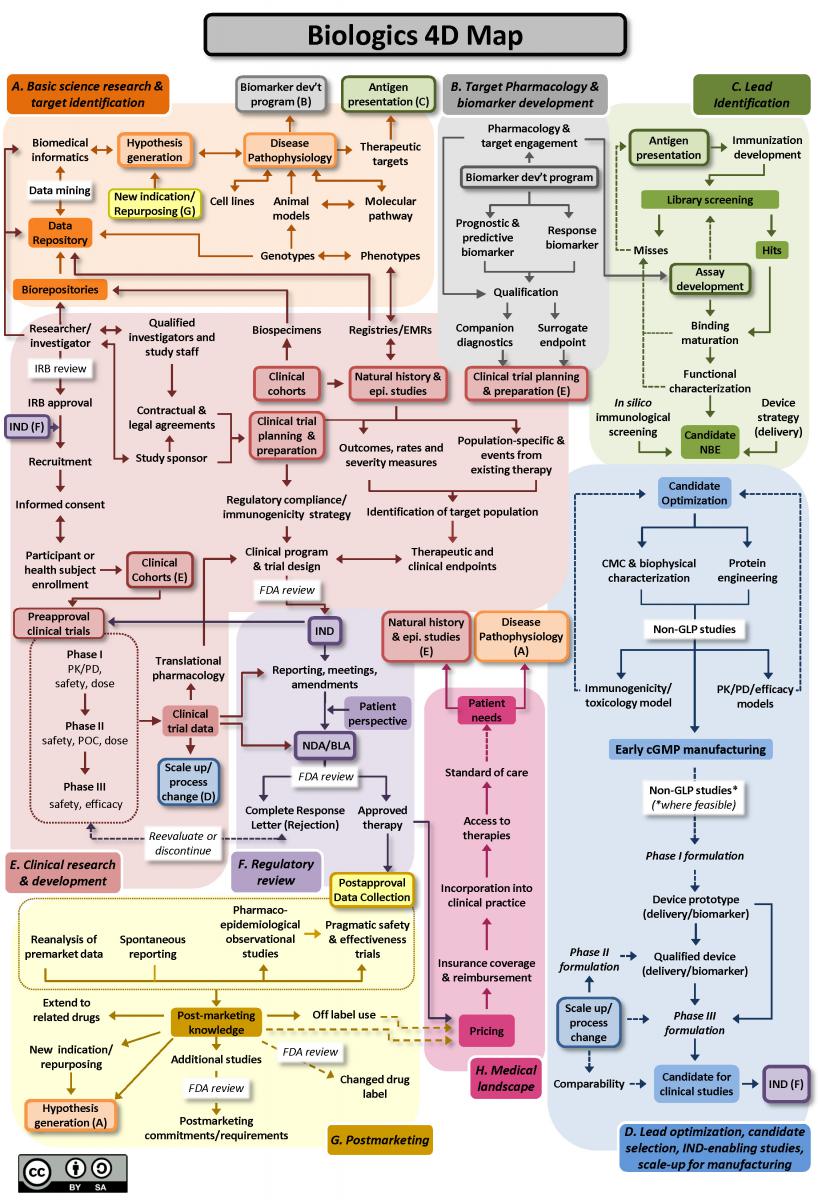
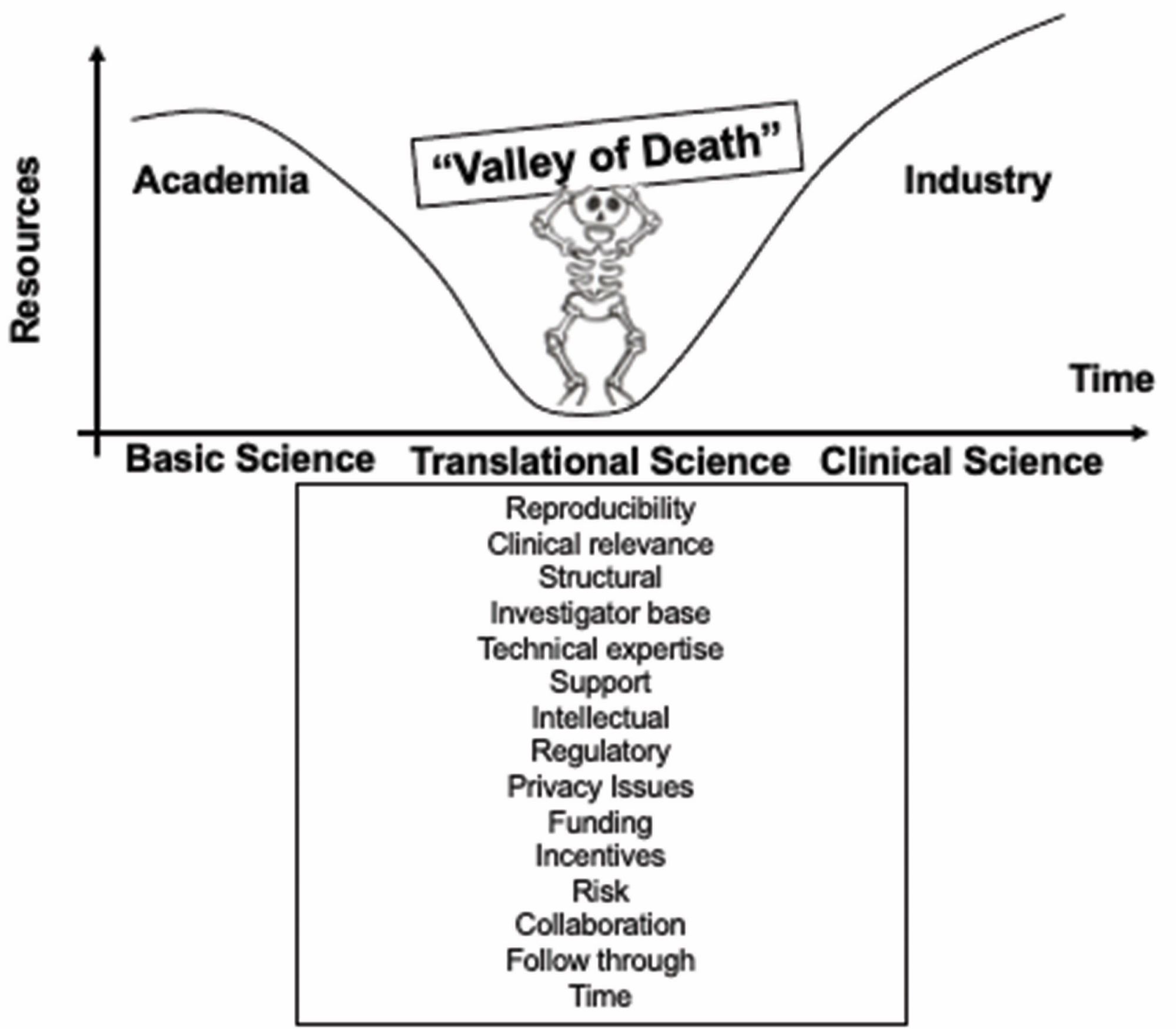
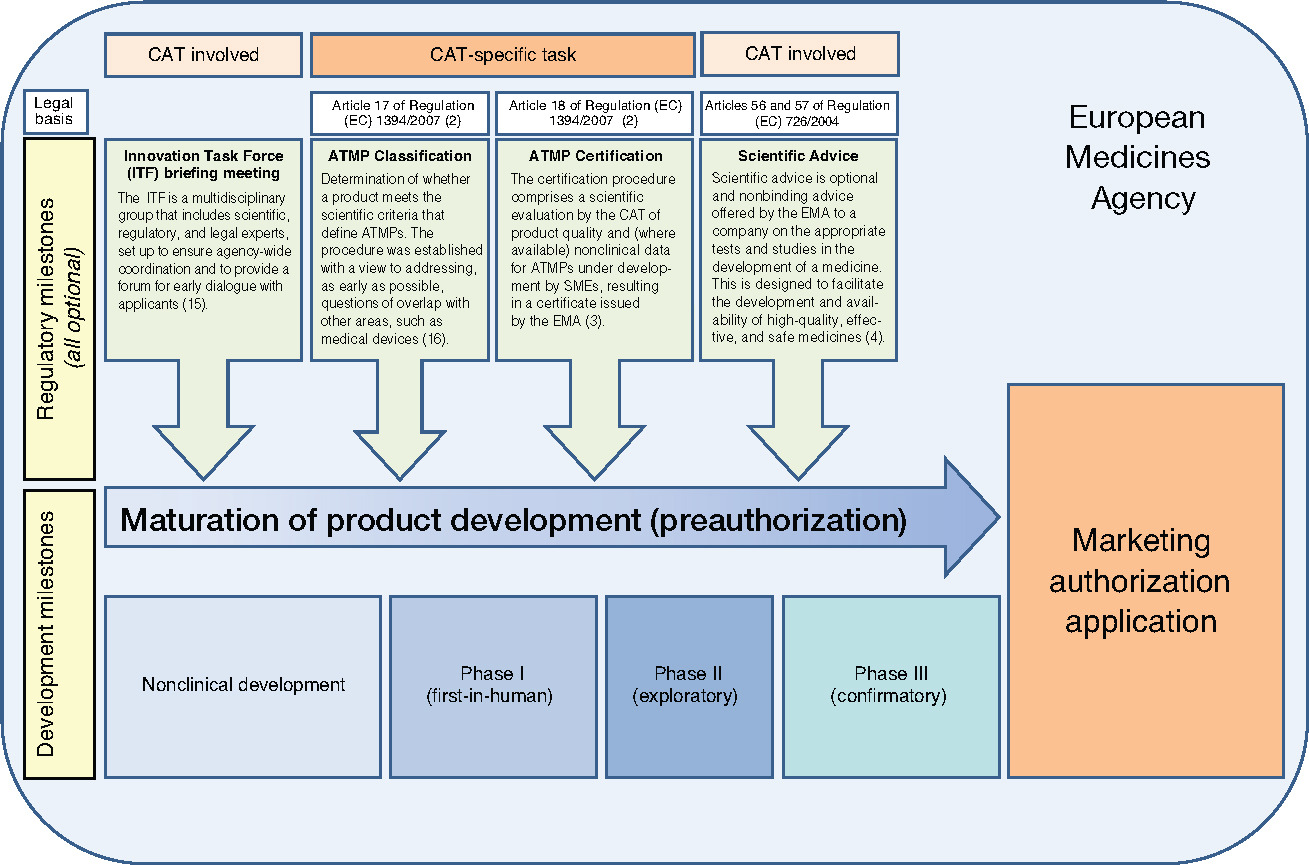
From “bench to bedside”, the mission of translational science is to bring predictivity and efficacy to the development and dissemination of intervention that improve human health (Austin CP, 2021). Translational science translates basic science discoveries into therapeutic applications. This is a progression from discovery research to pre-clinical studies defining targets, describing mechanisms of action and release criteria, to early stage human studies with primary endpoint(s) on safety, to more advanced and controlled clinical trials assessing efficacy on a large number of patients toward marketing authorisation applications. Exploratory studies performed during clinical trials accumulate data from drug product analysis and patient samples. Based on this data set, translational research can help to design next generation products and therapies.
In cell and gene therapies, the deep understanding of the pathophysiology allows to tackle the cause of the disease compensating for a missing factor. For example, a gene therapy for sickle cell which is due to a mutation of the beta globin gene was based on the addition of the normal version of this gene (lentiviral vector coding for beta globin). Another approach, was based on a deep understanding of the hemoglobin switching mechanism and on the reactivation of fetal hemobin (lentiviral vector coding for a shRNA against BCL11a).
Other drug development approaches are based on random screening of compounds or on empiricism. Some well known drugs like morphine for pain or aspirin for inflammation were developed without fully understanding the cause of the disease and the mechanism of action of the treatment. In gene and cell therapies, many challenges can prevent scientific breakthroughs to be translated into patient care: biological mechanisms underlying a potential clinical application have to be understood and converted into a therapeutic strategy not too complex to implement, pre-clinical models can be complicated to setup and may poorly mimic human physiology, manufacturing large scale batches of clinical grade products requires to control all the parameters that are critical to reach the targeted dose and to obtain the desired quality attributes of the product, a business model should be developed to cover the costs, to make the new therapeutic product accessible to patients and to allow investments.