DOI: https://journals.sagepub.com/doi/full/10.1089/hum.2022.29225.abstracts
Help and support
Help and support
- FAQ's
- Glosario
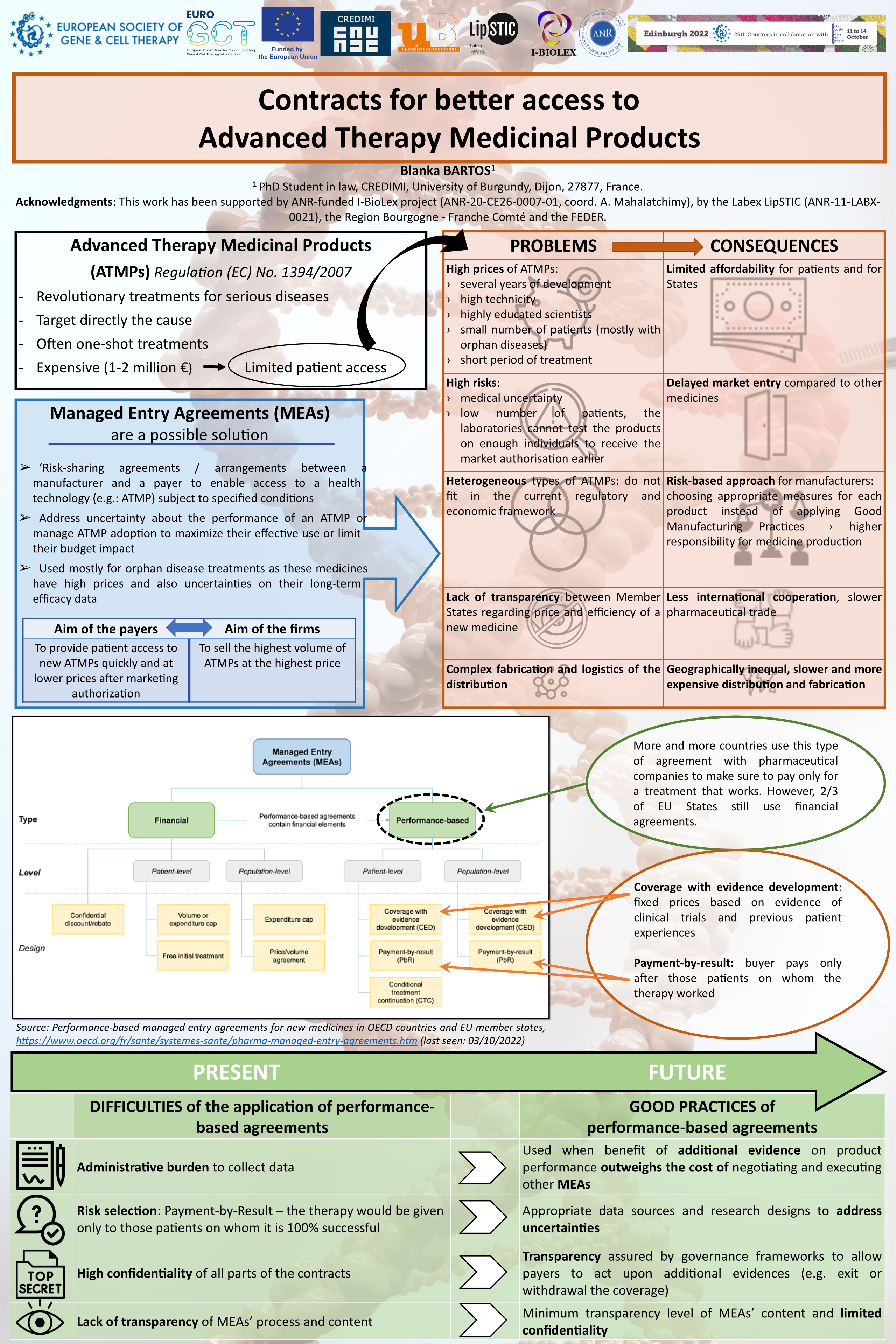
The greater part of ATMPs is manufactured in a customized way with high technicity to offer promising health benefits with mostly a one-shot treatment for incurable, often orphan diseases. The scope of use of these therapies is quite narrow and they also raise safety issues for patients. These are some of the reasons why ATMPs are extremely expensive and why they do not fit in the current regulatory and economic framework. If we want as many patients as possible to benefit from these medicines, we need to set a fair price for health insurance systems. On the basis of the Managed Entry Agreements (MEAs), the parties can negotiate on the performance of the product instead of signing a classic contract. The OECD distinguishes between two main types of agreements: financial and performance-based MEAs. Focusing on the latter, this paper will highlight its potential to facilitate access to ATMPs. The ‘‘coverage with evidence development (CED) contracts’’ allow treatment to be temporarily covered by the payer while a study evaluates its performance. Based on the results, coverage can be continued, withdrawn or extended, or prices are adjusted. The ‘‘pay-for-results contract’’ considers the uncertainty of results and organizes a ‘‘satisfied or reimbursed’’ policy. Here, the risk of innovation seems to lie with the laboratory and not with the community. Unfortunately, confidentiality is a barrier to evaluating objectively the strengths and weaknesses of these agreements. Greater transparency and an extended collaboration between States could help them negotiate better with drug laboratories.
The pdf file of this poster can be downloaded from the attachments section at the bottom of this page
Blanka Bartos, PhD Student in law, CREDIMI, Univ. of Burgundy, Dijon, France