Therapy classification determines the legal mechanisms that govern the regulation of gene and cell therapies to ensure the quality, safety, and efficacy of these therapies on the EU market.
Help and support
Help and support
- FAQ's
- Glossário
Therapy classification determines the legal mechanisms that govern the regulation of gene and cell therapies to ensure the quality, safety, and efficacy of these therapies on the EU market.
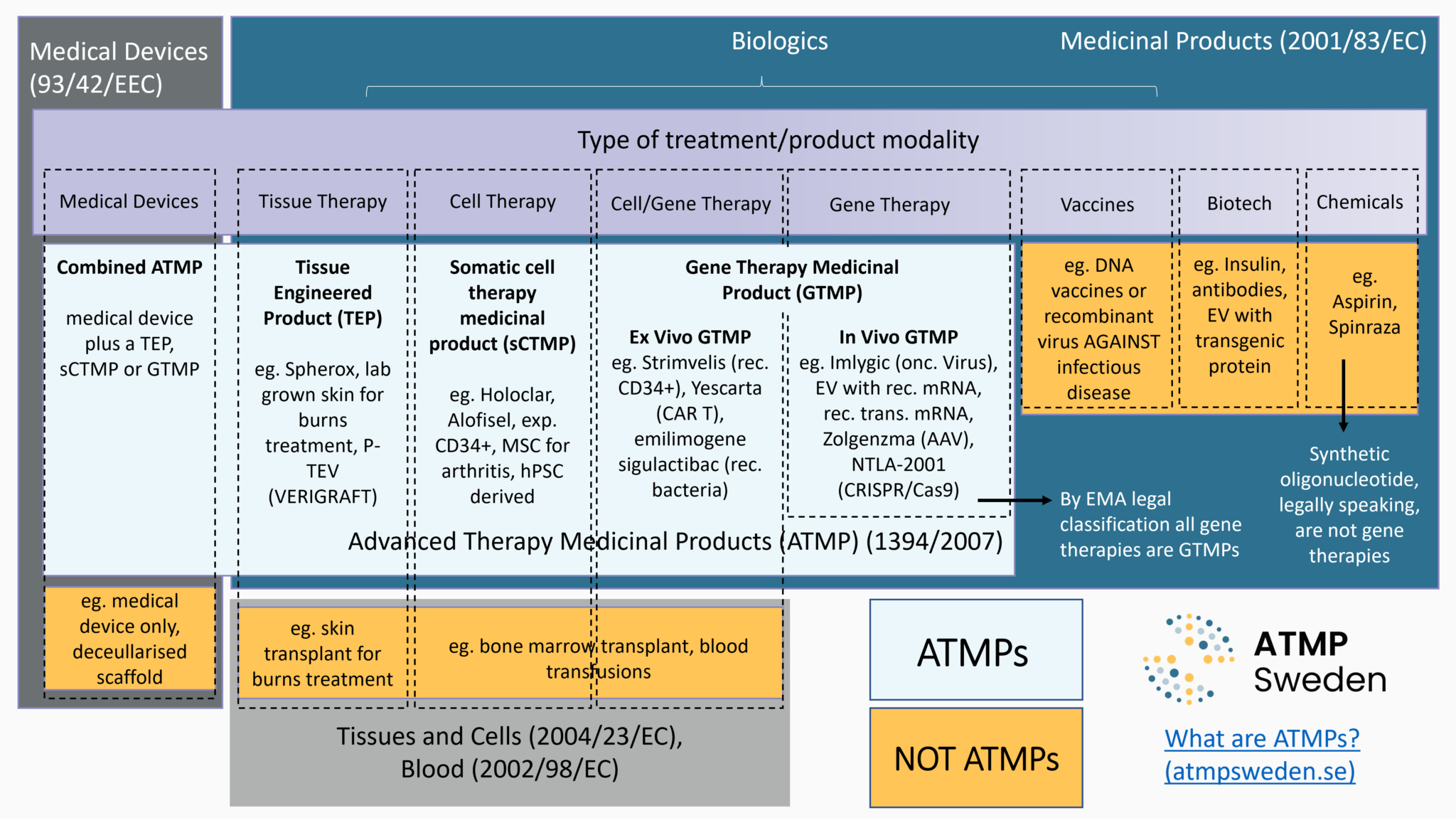
The first step regarding regulatory aspects in therapy development is to define the product being developed, namely its legal classification. Is it a medicinal product, a medical device, or cells or tissues used for therapeutic purposes? If it is a medicinal product, is it an Advanced Therapy Medicinal Product (ATMP), an orphan medicinal product and/or a medicinal product for paediatric use? The legal classification determines the applicable regulatory framework, i.e., the subsequent requirements that the product must meet, and the relevant procedures leading to commercialization, such as Marketing Authorisation (MA) for Advanced Therapy Medicinal Products (ATMPs). See also: Commercialisation.
The law (mainly European Union law but also national laws) provides legal definitions for each type of these products. Different criteria have been established to distinguish which type of gene and cell therapy are being developed and consequently which set of regulation applies both for market access and for reimbursement decisions. The main criteria are:
Despite these legal definitions and criteria, it remains difficult to know exactly what kind of gene and cell therapy is being developed according to the law. It is recommended for developers to contact regulators at the national or European level and reimbursement bodies as early as possible.
The Committee for Advanced Therapies (CAT) at the European Medicines Agency (EMA) is particularly involved regarding the legal classification of Advanced Therapy Medicinal Products in the European Union. In accordance with EU regulation (EC) n°1394/2007 on ATMPs, EMA has established the ATMP classification procedure to help developers clarify whether their product is an ATMP or not, and the applicable regulatory framework. CAT at EMA issues scientific recommendations based on whether the referred product meets the scientific and regulatory criteria defining ATMPs. The summaries of these recommendations, included the provided justifications are published on EMA website: See Scientific recommendations on classification of advanced therapy medicinal products).
ATMP is a type of gene and cell therapy, the development of which is particularly supported by European Union law both for public health (high level of human health protection in the EU) and economics (Good functioning of the internal market and competitiveness) objectives. There are several regulatory pathways for patients to access gene and cell therapy, including for ATMPs. ATMPs fall under the legal framework of medicinal products, especially biological medicinal products. ATMPs includes four sub-types of medicinal products based on genes, cells or tissues: gene therapy medicinal product (GTMP), somatic cell therapy medicinal product (sCTMP), tissue engineered product (TEP) and combined ATMP (cATMP). According to the EU law:
In this section, we will provide information regarding the different legal classifications of gene and cell therapy and the subsequent pathways to clinic.
Published: 29/01/2022
Last updated: 24/05/2025
Author:
Hsin-Yu Kuo, EuroGCT Project Manager - Research Information and Networks
Reviewed by:
Aurélie Mahalatchimy, EuroGCT WP4 Convenor, UMR 7318 DICE CERIC, Aix-Marseille University, CNRS, Aix-en-Provence- France