Help and support
Help and support
- FAQ's
- Glossar
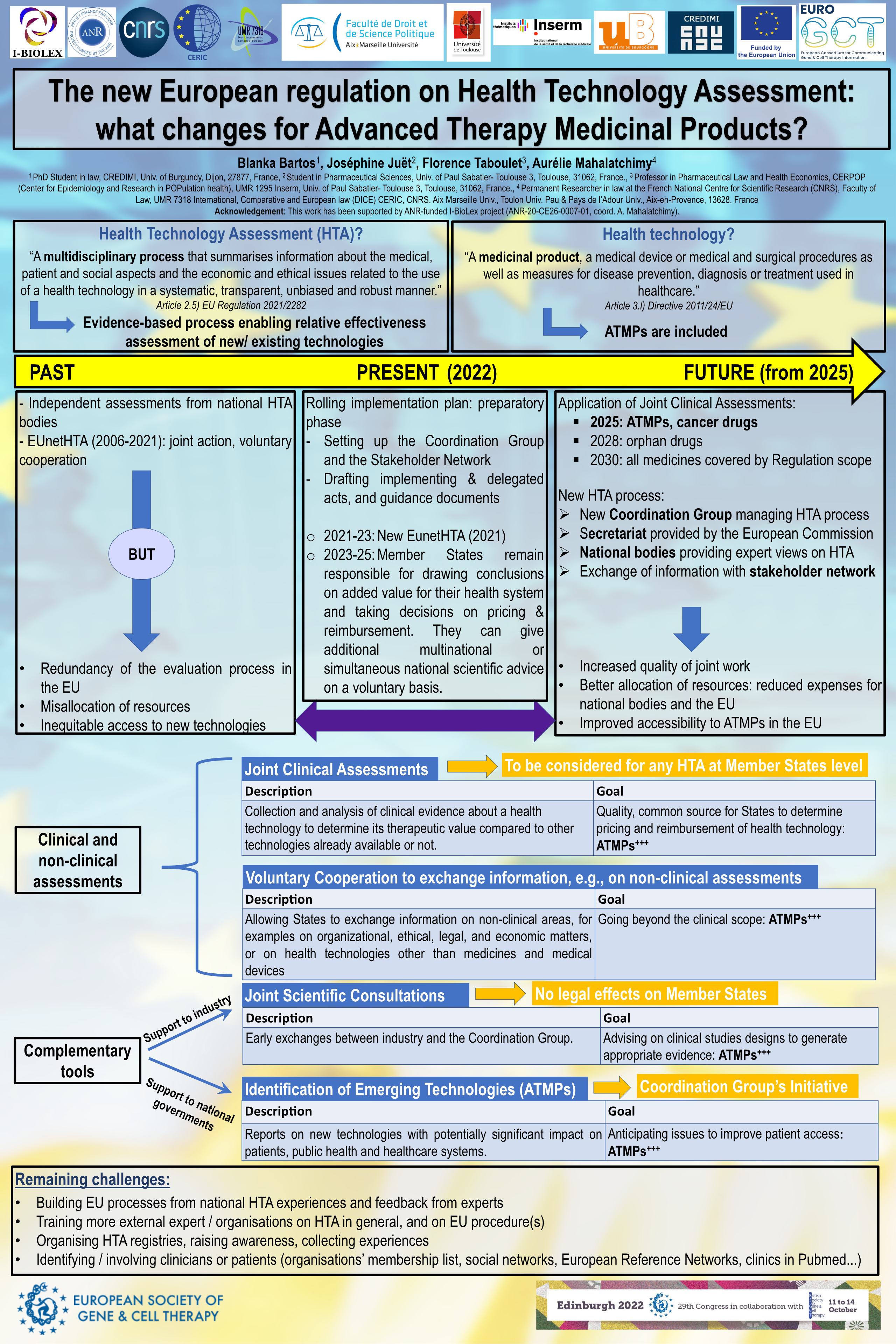
The new European regulation (EU) 2021/2282 on Health Technology Assessment (HTA), ‘‘a multidisciplinary process that summarises information about the medical, patient and social aspects and the economic and ethical issues related to the use of a health technology in a systematic, transparent, unbiased and robust manner’’, has been adopted on 15 December 2021. It ensures an efficient use of resources and strengthens the quality of common HTA across the European Union, because until now, the assessment has been realized independently by each Member State. It will thus contribute to the improvement of the availability of health innovative technologies for EU patients that has been widely highlighted by the Council of the European Union and the European Parliament. It mainly establishes a Member State Coordination Group on HTA, a stakeholder network, whom the joint works and exchanges will be facilitated thanks to an IT platform. It also provides rules for joint clinical assessments, joint scientific consultations, horizon scanning for emerging technologies, further voluntary cooperation notably on non-clinical assessments on health technologies, i.e. ‘‘a medicinal product, a medical device, or medical and surgical procedures as well as measures for disease prevention, diagnosis or treatment used in healthcare’’. The regulation shall apply progressively from 2025 to different types of health technologies, Advanced Therapy Medicinal Products (ATMPs) being the first targeted with cancer medicines. This poster will discuss the expected improvements for patients’ access to ATMPs regarding the forthcoming implementation of this new regulation.
The pdf file of this poster can be downloaded from the attachments section at the bottom of this page
Blanka Bartos, PhD Student in law, CREDIMI, Univ. of Burgundy, Dijon, France
Joséphine Juët, Student in Pharmaceutical Sciences, Univ. of Paul Sabatier - Toulouse 3, Toulouse, France
Florence Taboulet, Professor in Pharmaceutical Law and Health Economics, CERPOP (Center for Epidemiology and Research in POPulation health), UMR 1295 Inserm, Univ. of Paul Sabatier - Toulouse 3, Toulouse
Aurélie Mahalatchimy, EuroGCT Deputy coordinator and WP4 leader; UMR 7318 DICE CERIC, Aix-Marseille University, Centre National de la Recherche Scientifique, Marseille, Provence-Alpes-Côte d’Azur, France