Help and support
Help and support
- FAQ's
- Glosario
Medicinal products based on genes, cells and tissues of human or animal origin are being developed to treat severe diseases that conventional treatments have failed to treat or that have no available and satisfactory treatments, such as rare, genetic, or neurodegenerative diseases.
The risks they raise as innovative and complex medicines but also the therapeutic promises they offer have led to their specific regulation as Advanced Therapy Medicinal Products (ATMPs) in the European Union (EU). Indeed, they are especially submitted to the centralised procedure of Marketing Authorisation (MA) issued by the European Commission. This procedure aims not only to ensure high quality, safety and efficacy for ATMPs with a positive risk-benefit balance to be commercialised, but also a wide access to these medicines for EU patients. Nevertheless, this procedure alone is not always sufficient to allow the commercialisation of ATMPs, and many of them require expediting pathways or regulatory support schemes for innovative medicines to be commercialised. From the adoption of the EU regulation, 26 ATMPs have been granted a MA in the EU. Although there has been a clear increase in approvals from 2018, MA has been withdrawn or not renewed for 7 of them.
Starting from the centralised procedure as the strongest and first legal procedure for the widest and safest patients access to these innovative medicines, this poster will provide an overview of the European MA expediting pathways and of their use, for ATMPs to be accessible, and discuss the challenges of their quick but safe commercialisation.
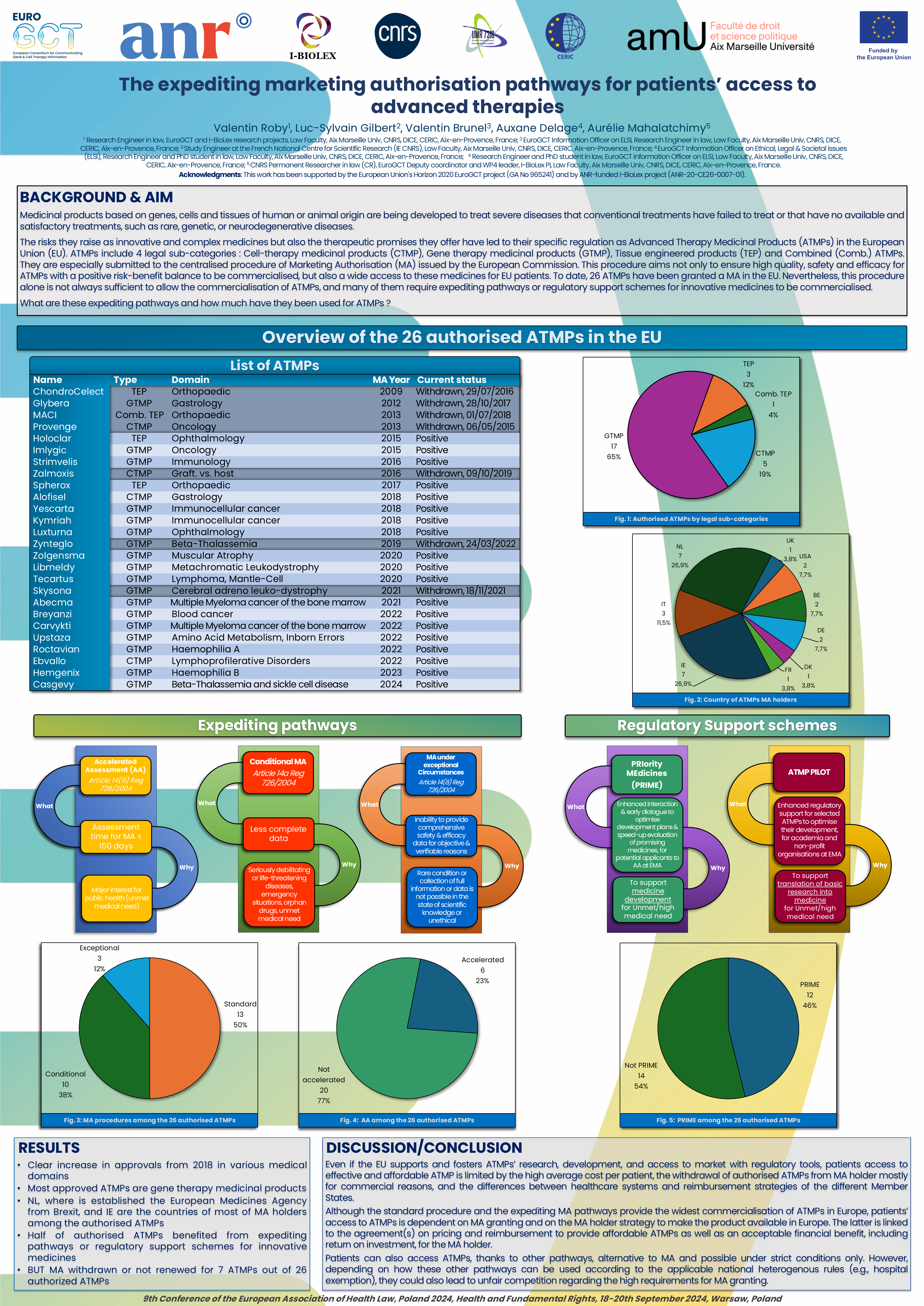
The pdf file of this poster can be downloaded from the attachments section at the bottom of this page
Valentin Roby, EuroGCT information officer on Ethical, Legal and Societal Issues
Luc-Sylvain Gilbert, EuroGCT Information Officer on Ethical, Legal & Societal issues, Aix-en-Provence, France
Valentin Brunel, Research engineer, Maison des Sciences de l’Homme SUD, Institut de Recherche pour le Développement, Montpellier, France
Auxane Delage, EuroGCT Information Officer on Ethical, Legal & Societal issues, Aix-en-Provence, France
Aurélie Mahalatchimy, EuroGCT Deputy coordinator and WP4 leader; UMR 7318 DICE CERIC, Aix-Marseille University, Centre National de la Recherche Scientifique, Marseille, Provence-Alpes-Côte d’Azur, France