Key resources for gene and cell therapy developers in the UK context are listed here:
Help and support
Help and support
- FAQ's
- Glosario
Key resources for gene and cell therapy developers in the UK context are listed here:

Key resources for gene and cell therapy developers in the UK context from the Medicines and Healthcare products Regulatory Agency (MHRA).

Guide published by UK Cell and Gene Therapy Catapult (CGT Catapult). Follow the link below to read this guide and more resources by CGT Catapult:
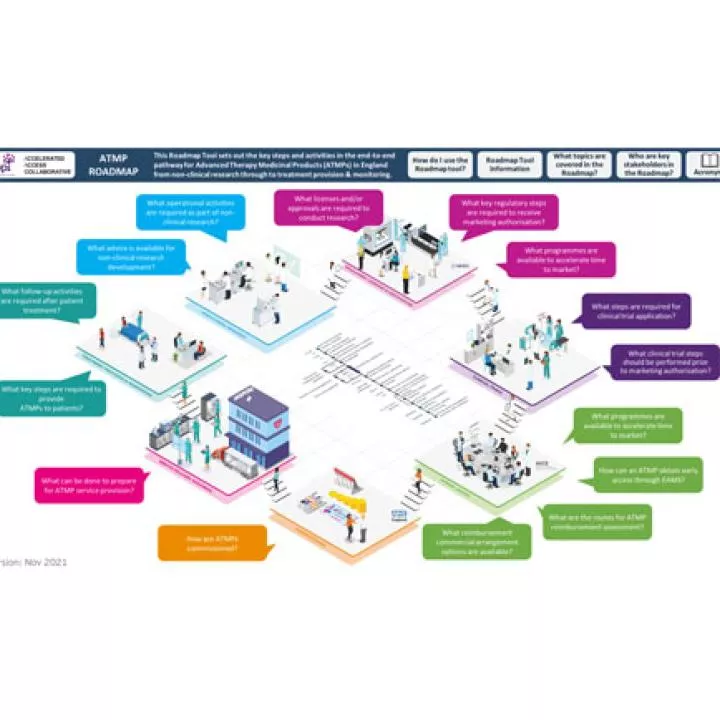
ABPI published a roadmap for ATMPs in UK, setting out key steps from non-clinical research through patient treatment & monitoring.

The Advanced Therapy Treatment Centres (ATTC) developed the Manufacturing and Preparation Toolkit to provide expert guidelines for manufacturing ATMPs. Learn more about this toolkit and additional resources developed by ATTC:

NIHR's interactive routemap to help understand the UK Clinical Trial Regulations
UKRI's interactive reference tool for the UK medicines sector, mapping and categorising organisations engaged in or supporting the end-to-end supply chain for medicines.