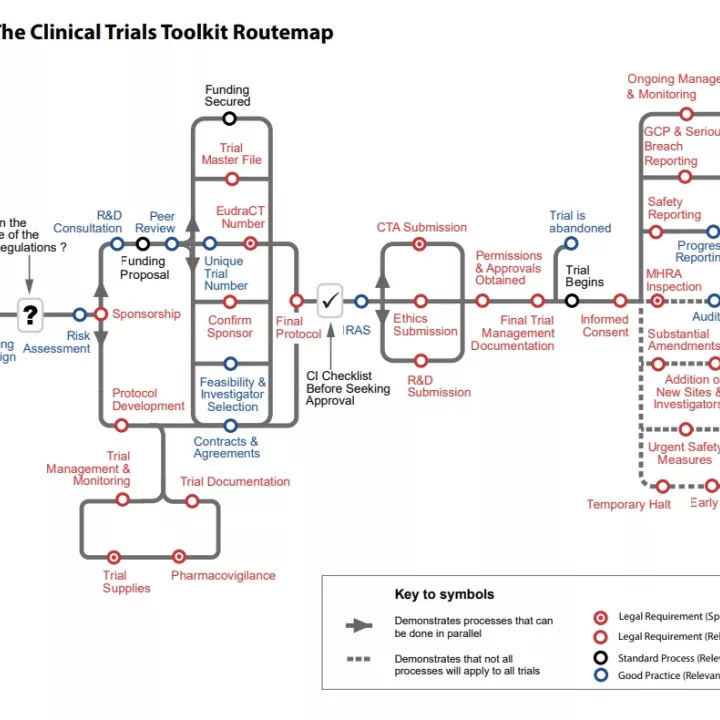
National Institute for Health Research (NIHR) designed the Clinical Trials Toolkit to help users understand the UK Medicines for Human Use (Clinical Trials) Regulations.
The toolkit is an interactive colour-coded routemap. Users can navigate through the legal and good practice arrangements surrounding setting up and managing a Clinical Trial of an Investigational Medicinal Product (CTIMP).
The routemap can be viewed online or downloaded as a PDF.