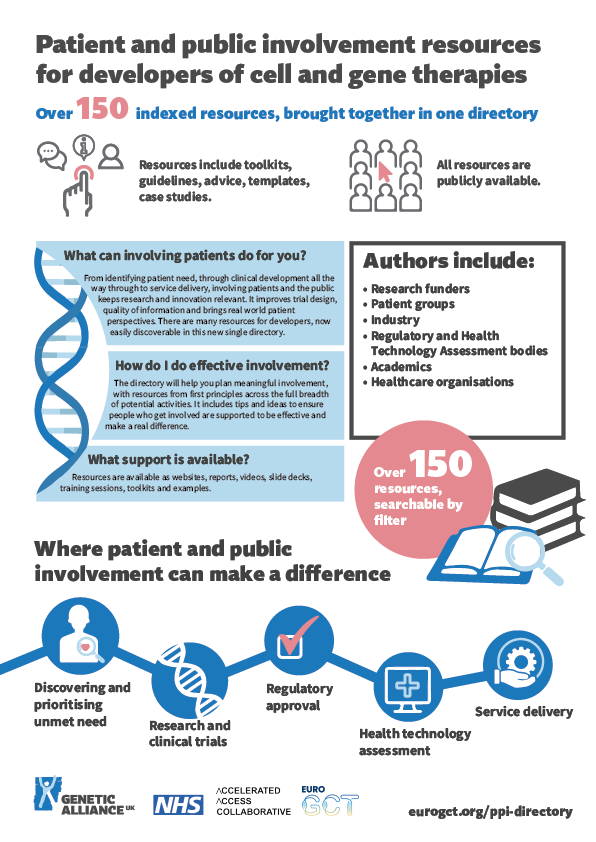
What is the PPI Directory?
The directory brings together over 150 indexed patient and public involvement resources for developers of cell and gene therapies. The resources include toolkits, guidelines, advice, templates and case studies. All the entries categorised as 'resources' are publicly available.
Who developed the directory?
The authors of the resources include research funders, patient groups, industry, regulatory and health technology assessment bodies, academics and healthcare organisations.
The directory is an output of the Advanced Therapy Medicinal Products Patient and Public Involvement and Engagement Working Group (ATMP Engage). The research, collection and indexing work was carried out by Genetic Alliance UK and commissioned by the Accelerated Access Collaborative. Genetic Alliance UK also produced a report that provides a brief guide to using the directory, outlines how it was developed and describes the gaps in available resources that became apparent. The report can be downloaded from the attachments section of this page. Following the launch of the online interface, EuroGCT will host and maintain the directory.
What can involving patients do for you?
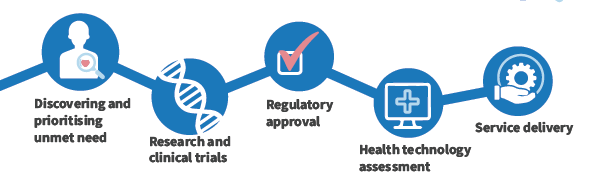
From identifying patient need, through clinical development all the way through to service delivery, involving patients and the public keeps research and innovation relevant. It improves trial design, quality of information and brings real world patient perspectives.
How do I do effective involvement?
This directory will help you plan meaningful involvement, with resources from first principles across the full breadth of potential activities. It includes tips and ideas to ensure people who get involved are supported to be effective and make a real difference.