This section contains key resources on ATMP development by medicines agencies, and existing complementary work produced by other organisations.
This section contains key resources on ATMP development by medicines agencies, and existing complementary work produced by other organisations.

Key European Medicines Agency (EMA) resources for cell and gene therapy development.

ATMP Sweden has compiled supporting resources and reports regarding ATMP development. Learn more about ATMP Sweden's Regulatory Guide and additional resources:

EJP RD's searchable reference library of resources in rare disease translational medicine. Learn more about this toolbox and other EJP RD resources:

The Alliance for Regenerative Medicine (ARM) and partners have produced A-Gene and A-Cell, case study-based guides to integrating Quality by design (QbD) principles in gene therapy and cell therapy Chemistry Manufacturing & Control (CMC) programs. Read about these projects and addiitonal ARM resources:
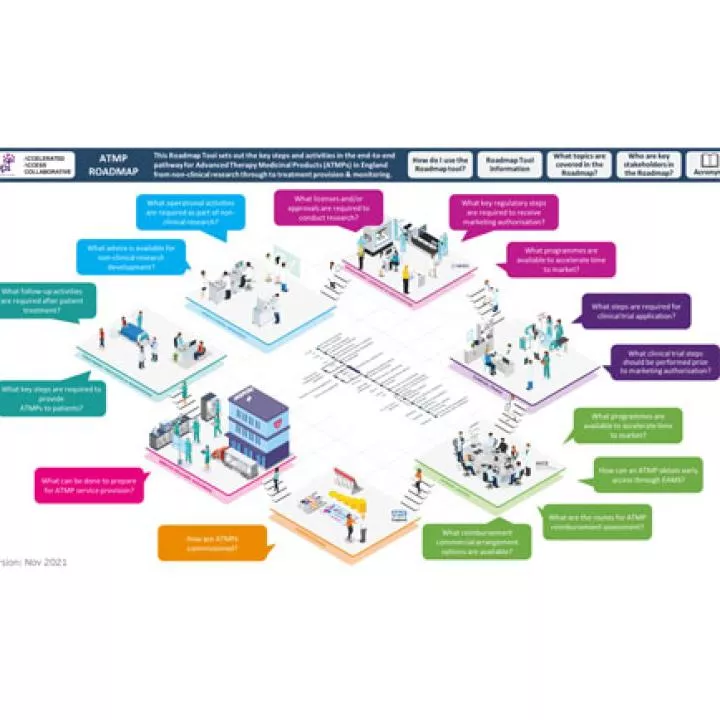
The Association of the British Pharmaceutical Industry (ABPI) has developed an ATMP roadmap, setting out the key steps in the end-to-end pathway for ATMPs in England from non-clinical research through to patient treatment & monitoring. Learn more about this roadmap and other relevant resources developed by ABPI:

See resource developed by EATRIS, including education and training initiatives for the next generation of ATMP professionals, patient engagement and more:

A patient-focused guidebook by the International Rare Diseases Research Consortium (IRDiRC) on developing drugs or therapies for rare disease indications.

The Advanced Therapy Treatment Centres (ATTC) developed the Manufacturing and Preparation Toolkit to provide expert guidelines for manufacturing ATMPs. Learn more about this toolkit and more from ATTC:

The National Center for Advancing Translational Sciences (NCATS) PaVe-GT pilot project aims to increase the efficiency of clinical trial startup by using the same gene delivery system and manufacturing methods for multiple rare disease gene therapies. Learn more about this project and additional resources developed by NCATS:

The Restore project develops technologies that will enable the reproducible and economically feasible manufacturing of Advanced Therapies.
Read about the roadmaps, reports, factsheets produced by this project:

A collection of key resources for gene and cell therapy developers in the UK context
A Report published by the Belgian Health Care Knowledge Centre (KCE) on academically developed ATMPs in Belgium.

The ATMP guidebook published by the Centre for Future Affordable & Sustainable Therapy Development (FAST) in Netherlands delivers essential guidance on European and Dutch regulations and provides a roadmap to navigate the complex ATMP development process.