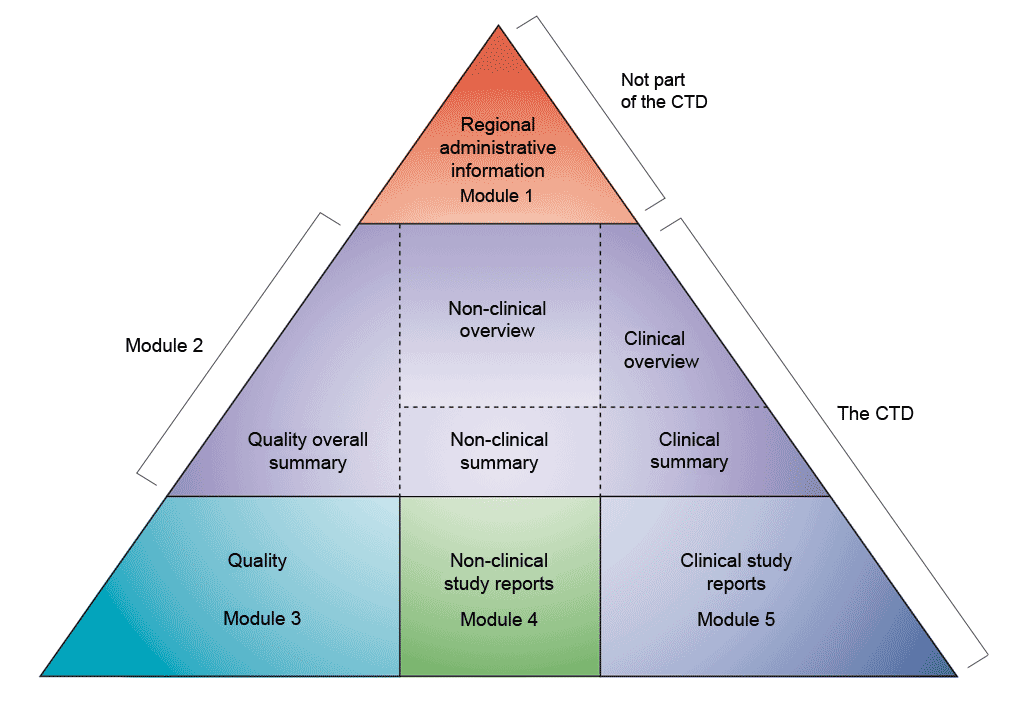
Based on the effect to be evaluated, the characteristics of the product to be tested and the criteria chosen for the assessment, different types of models can be used.
Different types of non-clinical or preclinical models can be distinguished based on their level of complexity, recapitulating the functions of a cell, of a tissue, of an organ or of the human body. The total number of cells in the human body is estimated at ≈36 trillion cells in the male, ≈28 trillion in the female, and ≈17 trillion in the child (PMID: 37722043). See figure 3. In addition, bacteria are about 38 trillion and are mostly living in the gut.
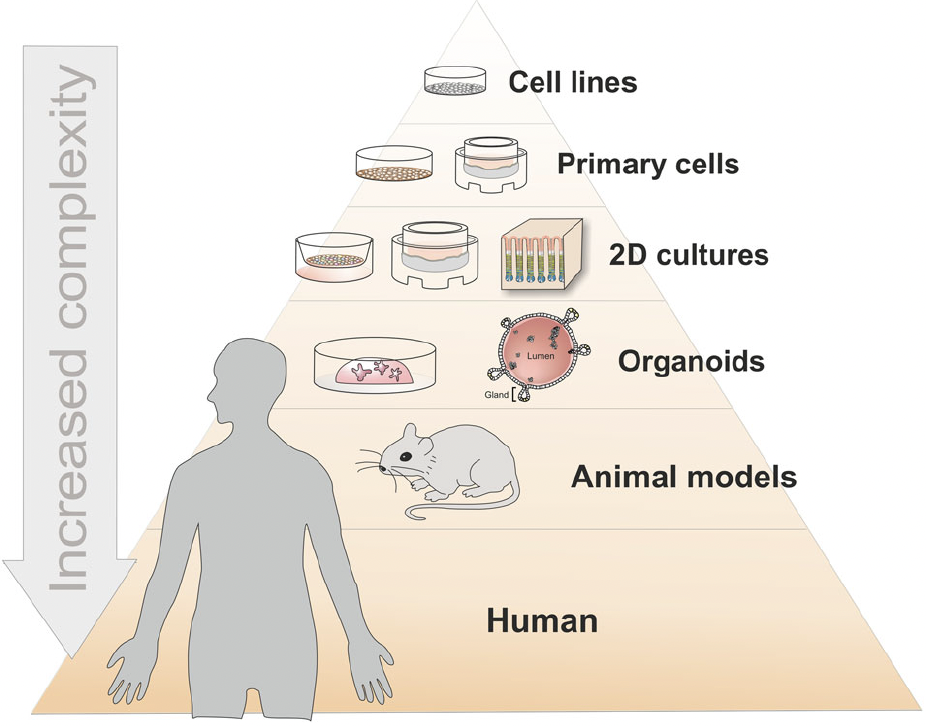
Figure 3: Complexity of current living models available for non-clinical studies
Organoids as host models for infection biology – a review of methods, Aguilar et al. Experimental & Molecular Medicine (2021) 53:1471–1482
Individual cells are practical but lack multicellular structure and miss the complexity of interactions between cells. Organoids fill the gap between monotypic cells and complex organisms. The term “organoid” has been used in the past to describe the histological characteristics of tumors or other tissues and generally meant “resembling an organ”. Organ-on-chip devices can recapitulate vascular flow, tissue–tissue interactions, and organ-relevant mechanical movements. Animal models recapitulate the complexity of organisms such as mammals without perfectly mimicking the human physiology.
- In vitro models such as human cells in liquid culture, on feeder cells (2D), in organoids (3D), or on chips
Primary cells and cells lines are used in liquid culture to perform preclinical studies such as biochemistry, cell biology and immunology assays. Cultures on feeder cells benefit from growth factors and interactions with the layer of feeders supporting the growth and the differentiation of the cells of interest for preclinical studies.
Organoids are miniature 3D models mimicking organs or tissues, enabling scientists to investigate the causes of diseases and test new treatments. e.g. brain organoids to evaluate cellular therapies (Brain Organoids to Evaluate Cellular Therapies, García-Delgado AB, Campos-Cuerva R, Rosell-Valle C, Martin-López M, Casado C, Ferrari D, Márquez-Rivas J, Sánchez-Pernaute R, Fernández-Muñoz B. Brain Organoids to Evaluate Cellular Therapies. Animals (Basel). 2022 Nov 15;12(22):3150) or organoid models for assessing cancer immunotherapy (Breakthroughs and challenges of organoid models for assessing cancer immunotherapy: a cutting-edge tool for advancing personalised treatments, Wang, Q., Yuan, F., Zuo, X. et al. Breakthroughs and challenges of organoid models for assessing cancer immunotherapy: a cutting-edge tool for advancing personalised treatments. Cell Death Discov. 11, 222 (2025)). See figure 4.
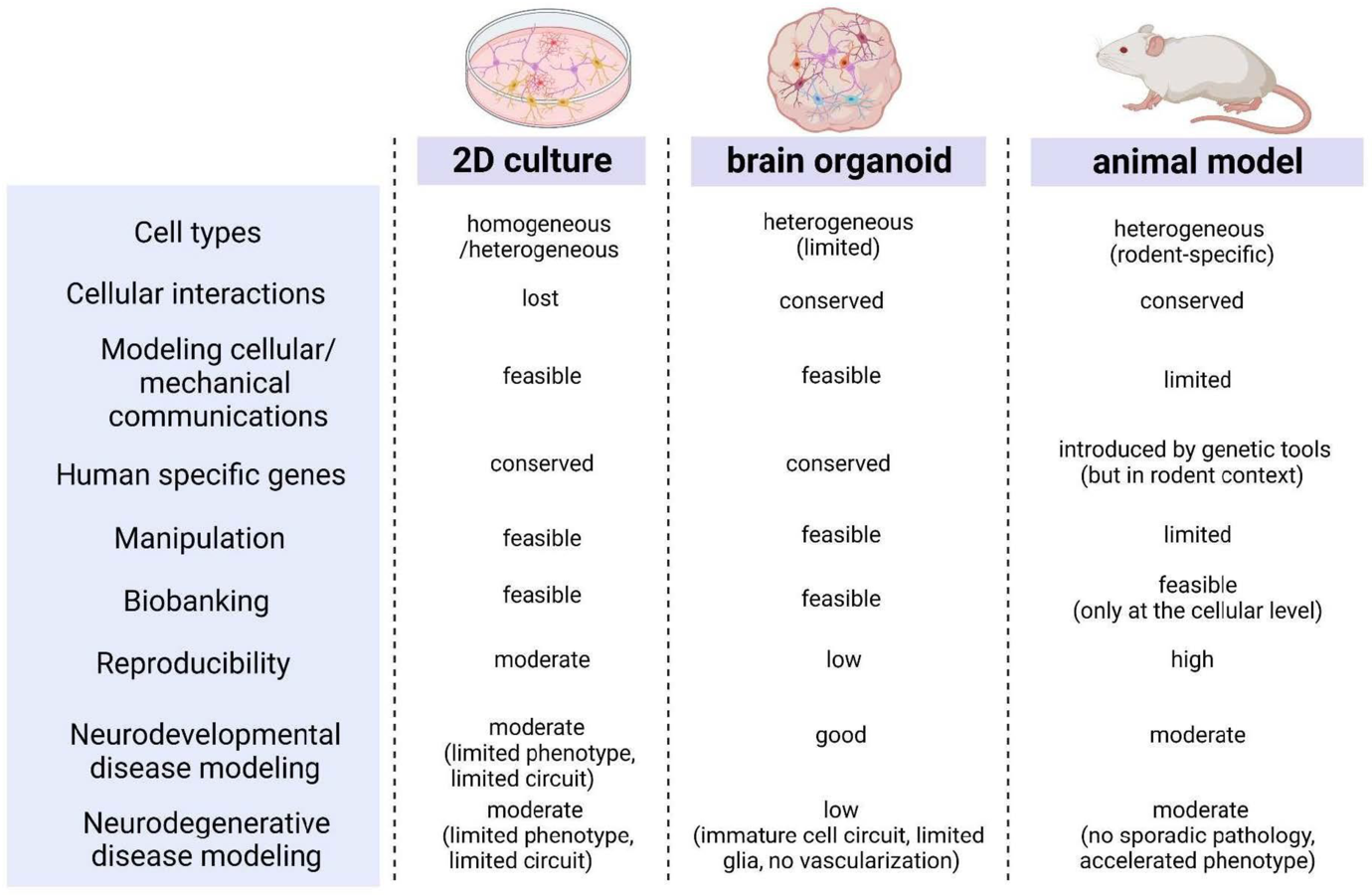
Figure 4: Two-dimensional (2D) cell culture, three-dimensional (3D) brain organoid models. Int. J. Mol. Sci. 2023, 24, 12528. Application of Human Brain Organoids—Opportunities and Challenges in Modeling Human Brain Development and Neurodevelopmental Diseases.
The comparison of characteristics among two-dimensional (2D) cell culture, three-dimensional (3D) brain organoid models, and animal models allows to choose the model the most suitable model for a given study. It should be simple enough to be well described by the parameters of the experiment and relevant for future applications on the human body. Brain organoids become alternatives to animal models or classical 2D culture systems in experiments and their significance as valuable models for studying brain developmental disorders is increasing.
Cells on chips aim to miniaturize and complete assays on lab-on-a-chip systems allowing control of culture conditions and measurements of biomarkers more precisely e.g. pancreas on chip.
Organoids as preclinical models of human disease: progress and applications, Chen B, Du C, Wang M, Guo J, Liu X. Organoids as preclinical models of human disease: progress and applications. Med Rev (2021). 2024 Mar 14;4(2):129-153.
Pancreatic islet organoids-on-a-chip: how far have we gone?, Yin J, Meng H, Lin J, Ji W, Xu T, Liu H. Pancreatic islet organoids-on-a-chip: how far have we gone? J Nanobiotechnology. 2022 Jun 28;20(1):308.
- Ex vivo models such as perfused organs or organ-on-chips
Organs perfused ex vivo offer an excellent experimental platform for studying organ physiology and testing new treatments.
Ex-Vivo Human-Sized Organ Machine Perfusion: A Systematic Review on the Added Value of Medical Imaging for Organ Condition Assessment, Van Der Hoek JL, Krommendijk ME, Manohar S, Arens J and Groot Jebbink E (2024)
Organs-on-chips are systems containing engineered or natural miniature tissues grown inside microfluidic chips. To better mimic human physiology, the chips are designed to control cell microenvironments and maintain tissue-specific functions.
A guide to the organ-on-a-chip, Leung, C.M., de Haan, P., Ronaldson-Bouchard, K. et al. Nat Rev Methods Primers 2, 33 (2022).
- In vivo models such as animal models mimicking human disease conditions
Animal models mimicking human disease conditions are very useful at the preclinical stage before embarking on a clinical trial. In gene therapy, for instance, they are useful in the assessment of variables related to the use of viral vectors such as safety, efficacy, dosage and localization of transgene expression.
Contemporary Animal Models For Human Gene Therapy Applications, Gopinath C, Nathar TJ, Ghosh A, Hickstein DD, Nelson EJR. Curr Gene Ther. 2015;15(6):531-40.
Small animals such as mice are often used to develop advanced therapies related to hematology or immunology, e.g. development cellular immunotherapies like CAR-T cells. Transgenic mice are generated to create models of human diseases. Immunodeficient and humanized mouse models are generated to test human cells in xenogeneic studies. Cellular therapies based on human hematopoietic stem cells (therapies for Adenosine Deaminase Deficient Severe Combined Immunodeficiency (ADA-SCID), transfusion-dependent thalassemia (TDT), Metachromatic Leukodystrophy (MLD), Sickle Cell Disease (SCD) … ) or immune cells (therapies for Multiple Myeloma (MM), B-cell Acute Lymphoblastic Leukemia (B-ALL), Acute Myeloid Leukemia (AML) …) are often tested in immunodeficient mice (nude mice, SCID mice, NOD SCID mice, NSG mice, NOG mice, BRGSF mice, NCG mice, etc…) or in humanized mouse models (usually generated to better recapitulate the development of human innate and adaptive immunity) before being assessed in clinical trials.
Larger animals such as dogs or non-human primate are used for preclinical studies on muscles and central nervous system, e.g. test of Adeno-Associated Virus (AAV) vectors to treat myopathies or neurodegenerative diseases.
In silico models are developed by computer programmers and data scientists and built with real world data sets such as clinical data, genomics, proteomics, transcriptomics … Artificial intelligence algorithms are often used to navigate big data sets and interact with end users like wet lab scientists and clinicians.
In silico regenerative medicine: how computational tools allow regulatory and financial challenges to be addressed in a volatile market, Geris L, Guyot Y, Schrooten J, Papantoniou I. Interface Focus. 2016 Apr 6;6(2):20150105.
The most advanced in silico models aim at becoming digital twins of patients and be used to accelerate the selection of drug candidates and the development of medicinal products. See figure 5.

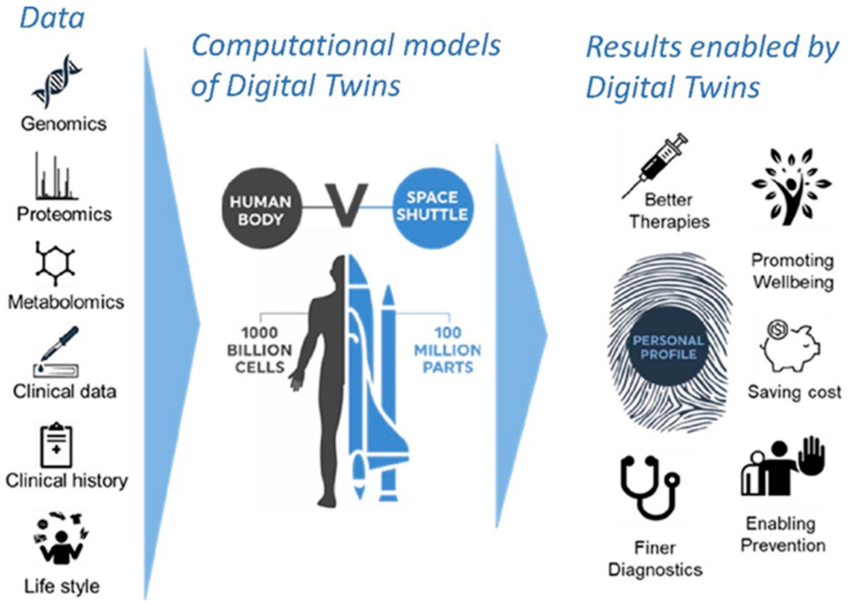
Figure 5: the concept of digital twins
Figures adapted from Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity, Lee S, Rauch J, Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. International Journal of Molecular Sciences. 2020; 21(3):1102.
Integrating molecular, clinical and imaging data through computational models will allow to generate digital twins of tissues, organs and patients. New treatments could be first tried on digital twin and the best option selected for clinical trials (Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity). Creating a digital twin of a patient is highly challenging. If 100 million parts have to be considered for a space shuttle, 1000 000 million cells should be considered for a human body.