
Help and support
Help and support
- FAQ's
- Słownik
CARTALLEU is a Phase 2 clinical trial to evaluate the efficacy and safety of Var-cel, a CAR-T therapy for relapsed or refractory adult acute lymphoblastic leukaemia. This confirmatory EU-based trial is being conducted to gain centralised approval in the EU via the European Medicines Agency (EMA), in academic centres from 5 different EU countries.

Chimeric antigen receptor T-cell therapy, or CAR-T therapy, is a personalised cell therapy that can help treat certain kinds of cancers.
CARTALLEU is a Phase 2 clinical trial to evaluate the efficacy and safety of Var-cel (varnimcabtagene autoleucel). Var-cel is a CAR-T therapy for relapsed or refractory adult acute lymphoblastic leukaemia (R/R ALL), a type of blood cancer that starts from white blood cells called lymphocytes in the bone marrow. ‘Relapsed or refractory’ means that the cancer has come back after a period of remission, or has stopped responding to other treatments.
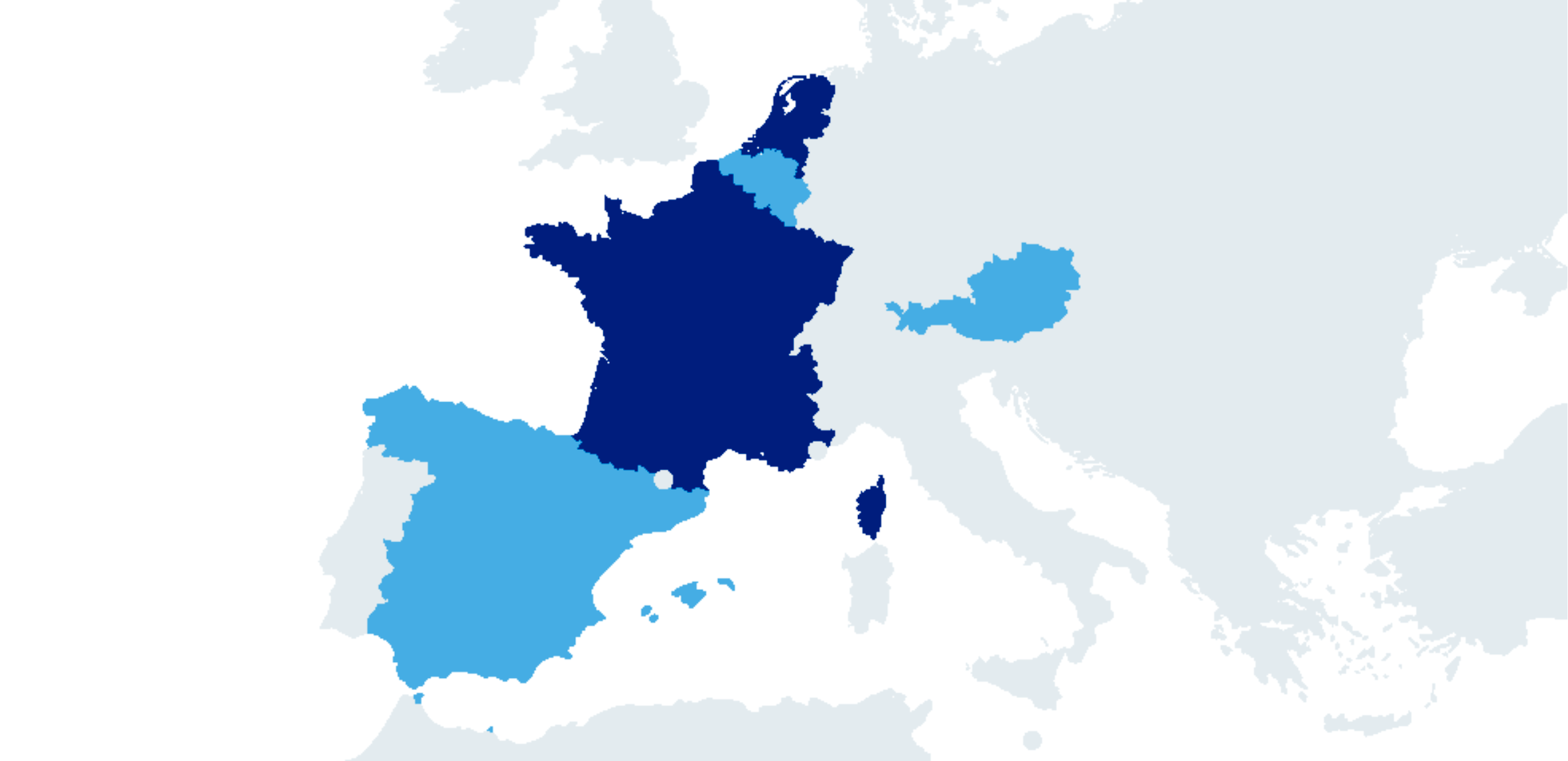
Var-cel was developed within academia and has been approved in Spain for the treatment of patients older than 25 years with R/R ALL, under the Hospital Exemption Clause. This confirmatory EU-based clinical trial is being conducted to gain centralised approval in the EU via the European Medicines Agency (EMA). The trial is being carried out in academic centres across 5 different EU countries: Spain, France, Belgium, Austria and The Netherlands.
We are working with Prof. Julio Delgado, Oncoimmunotherapy, FRCB-IDIBAPS/HCB, Barcelona (Spain). Our ultimate goal is to be able to offer this therapy at reduced cost.





CARTALLEU is enabled by ATTRACT, a collaborative research initiative jointly promoted by multiple European cancer charities which aims to accelerate therapy development for rare cancers. Our funders are Fundación científica Asociación Española Contra el Cáncer (Spain), Fondation ARC (France), Kom op tegen Kanker (Belgium), and the KWF Kankerbestrijding (The Netherlands).
Professor Julio Delgado recognized the immense potential of CAR-T therapy in 2011, but was concerned that the high prices of manufacturing would result in limited access, particularly as CAR-T is used to treat rare diseases. Julio saw an opportunity to produce CAR-T within hospitals, taking advantage of existing manufacturing facilities, and make it available for patients at cost price. However, he faced resistance, as this was seen as the role of pharmaceutical companies, and it was difficult to attract investors.
Crowdfunding, led by a leukemia patient, raised €1 million to treat the first 10 patients, generating data which made further trials possible. Since continuous clinical trials were unsustainable, Julio pursued nonprofit (academic) production, working within the EMA for 15 months to understand the relevant regulations.
National funding restrictions and cross-border treatment barriers made a centralised treatment center impossible, so CARTALLEU is now funded by multiple EU member states and patient groups.
Although ATMPs have transformed the treatment of some rare diseases, high production and research costs make them inaccessible for many patients. In addition, some approved treatments have been withdrawn from the market after the manufacturer decides they are no longer profitable. The EU introduced the principle of Hospital Exemption to regulate for ATMPs, allowing some treatments to be used without marketing authorisation under specific circumstances. You can learn more about EU Hospital Exemption below.